
(© sudok1 - stock.adobe.com)
HERSHEY, Pa. — A new study by researchers at Penn State finds a specific variation of white blood cells may induce death in brain cancer tissues. While that sounds like good news initially, researchers caution it may not be as positive as it appears. Prior research notes high levels of this kind of tissue death actually results in worse survival outcomes for patients battling aggressive glioblastomas, one of deadliest forms of brain cancer.
Scientists call this phenomenon of cancer tissue death necrosis. Researchers have speculated for some time that necrosis may be caused by a lack of oxygen due to blood loss as a consequence of rapid tumor growth. For this research, the team focused on the molecular processes underlying tissue death.
“Glioblastoma patients with higher degrees of necrosis have a poor chance of survival,” says Wei Li, assistant professor of pediatrics and biochemistry and molecular biology, in a university release. “We hope insight into the processes that drive this tissue death can help us develop new therapeutics to improve outcomes for these patients.”
Cell death’s link to cancer
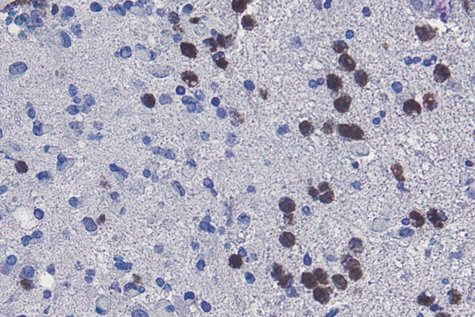
Li and his team discovered that ferroptosis, a type of regulated cell death, is responsible for tissue death. Then, examinations of glioblastoma tissue samples collected from animals revealed that neutrophils (a type of white blood cell) were indeed present in the same areas as the dead cancer cells.
To establish whether or not these cells are part of the tissue death process, researchers then lowered the amount of neutrophils in the animal models. Sure enough, that action ended up decreasing the amount of necrosis in the cancer models.
White blood cells were even isolated in a lab setting, exposed to cancer cells, and subsequently stopped the cancer cells from “thriving.”
“We confirmed our theory on the role of neutrophils in necrosis by evaluating glioblastoma patient data,” Li says. “A high number of neutrophils and the presence of genetic signals of ferroptosis were associated with pathological evidence of necrosis and predicted poor survival in patients.”
Dead cells still have something to say
Still, all of this left the lingering question: why does cell death appear to help tumor progression? In pursuit of answers, Li’s team studied glioblastoma patient data sets. Eventually, they discovered that dead cells release molecular signals that may aid the growth of tumor cells.
Researchers want future studies to focus on how these white blood cells arrive at cancer locations in the first place. Li hypothesizes that tissue damage inflicted by tumors draw the attention of white blood cells.
“If we can develop therapeutic approaches for preventing necrosis, there’s a chance those tumors might be less aggressive, which could be beneficial to glioblastoma patients,” Li concludes.
The study is published in Nature Communications.







